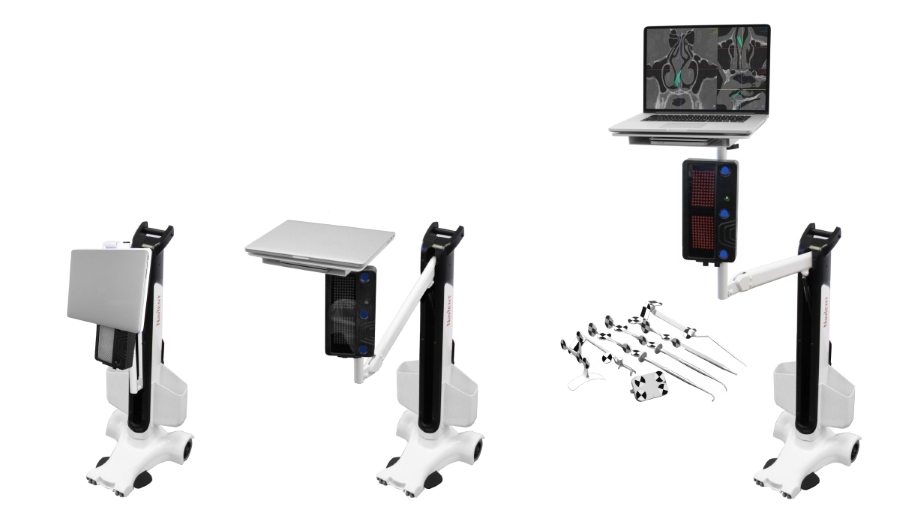
NaviENT brings Simplicity, Accuracy and Affordability to ENT Navigation Systems.
Toronto, CANADA – Nov 14, 2016
ClaroNav Kolahi Inc. (CKI) announced today that it has received CE Mark approval for the commercial sale of its NaviENT cranial and ENT navigation system in Europe. Over the coming months the company will offer the product for sale to hospitals, clinics and offices performing endoscopic sinus surgery and skull base surgery throughout Europe.
NaviENT shows the location of the tip of surgical instruments relative to surrounding anatomy, as seen in a pre-surgical CT scan, to guide surgical intervention. It enables surgeons to confidently make more informed decisions during transnasal surgery, such as when treating sinusitis or skull base tumors.
“NaviENT was designed in concert with rhinologists to develop the most user-friendly, accurate, reliable, and affordable navigation system on the market. It is clear that they have achieved their goals. In this case, using is believing.” said Peter Catalano, MD, FACS, FARS, Professor of Otolaryngology, Chief of Otolaryngology, St. Elizabeth’s Medical Center, Boston. “The system sets up fast, registration is complete in 2 minutes, and because it has reusable instruments, there are no per-case costs! Lastly, its simplicity speaks to its durability as no maintenance contracts are required.”
Surgical navigation can help surgeons in highly variable sinus anatomy to target specific anatomy and avoid critical areas. It would allow more complete dissection, obviating the need for revision surgery. It results in substantial cost saving by shortening length of hospitalization compared to non-navigated surgeries.
For more information about the NaviENT, please visit www.claronav.com/navient
About ClaroNav Kolahi Inc. (CKI)
CKI is a subsidiary of ClaroNav, a medical device hardware and software company headquartered in Toronto, Canada, with offices in the USA, Belgium, Italy and Singapore. ClaroNav and CKI are deeply committed to product quality and safety, and certified as compliant with ISO 13485, ISO 14971 and CMDCAS. CKI is dedicated to innovation and developing state-of-the-art cranial surgical navigation systems to enable surgeons to confidently make more informed decisions for better patient outcomes. For more information, visit www.claronav.com.