by ClaroNav | Sep 13, 2017 | Events, News
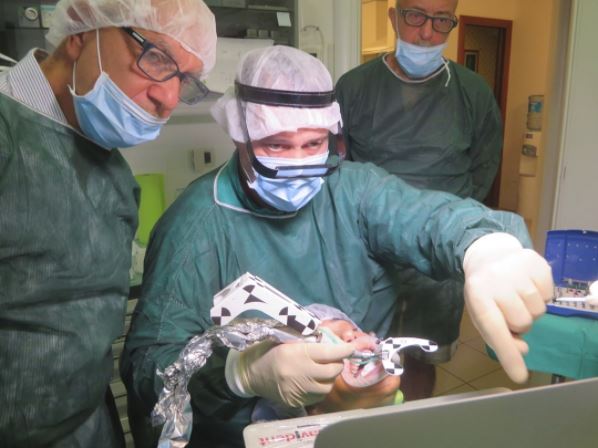
In Lucca, a new Navident Certification course took place. Dr. Gian Luigi Telara, Master Clinical Trainer oversaw the system use habilitation of Dr. Riccardo Bacco (Torino) and Dr. Alberto Borgioli (Cerreto Guidi – FI). Attendees were satisfied with the course...

by ClaroNav | Sep 6, 2017 | News, Press Release
Toronto, Ontario, Canada, September 6, 2017 ClaroNav Kolahi Inc. (CKI), a subsidiary of ClaroNav, announces that NaviENT, its Surgical Navigation System, received FDA 510(K) clearance. CKI is pleased to announce that it has received FDA 510(K) clearance from the Food...

by ClaroNav | Jul 20, 2017 | News
On July 7, the Tuscan Dentistry Institute of Prof. Ugo Covani and ClaroNav presented the first national meeting on topics related to the use of Navident, which saw the participation of the doctors Stefanelli, Mandelaris, Telara and Sartori as speakers. The meeting...

by ClaroNav | May 23, 2017 | News
Silicon Review recently named ClaroNav as one of 10 Fastest Growing Robotics Companies 2017. Our CEO, Doron Dekel, was interviewed by Silicon Review as a part of this series and you can read this feature here.

by ClaroNav | May 23, 2017 | News
Navident is a cover girl now (this is the “targeting perfection” visual we associate with Navident in our ads). Inside the IDS issue of Implants, there is a great case study by Dr. David Burgess of the UK. Dr. Burgess also documented a Navident case that you can view...






