Newsletter – Issue 1
Year: 2015
Issue: No. 1



PRODUCT UPDATES
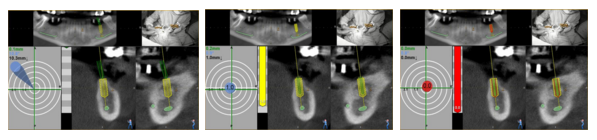
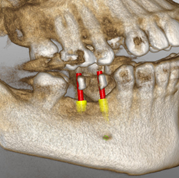 Just released: Navident’s first software update, version 1.1
Just released: Navident’s first software update, version 1.1
Key features added in Navident 1.1 include:
- Fully automated, more reliable, fiducial registration
- 3D view of the plan
- Combo guidance indicator
- Improved tracking reliability with a new high-dynamic range (HDR) mode
- Planning-only version for installing on any computer running Windows 7 or 8
Request an online demo here
Regulatory clearance update
Navident received Health Canada clearance as a class 3 device on 29 May 2014.
 We submitted a technical file to obtain a CE mark (class IIa) on 12 November 2014, and are currently interacting with our registrar to address minor documentation issues. We are hopeful that Navident will be cleared for sale in Europe in time for the IDS show.
We submitted a technical file to obtain a CE mark (class IIa) on 12 November 2014, and are currently interacting with our registrar to address minor documentation issues. We are hopeful that Navident will be cleared for sale in Europe in time for the IDS show.
![]()
We submitted our FDA 510(k) clearance application (class II) on 23 December 2014, and are waiting to hear back. Typical delay from FDA 510(k) submission to clearance is 3-6 months.
CORPORATE UPDATES
2014 Year-End Review: Thank you for a successful year!
 This has been a busy year, one full of exciting changes and achievements. Navident product developments have brought major improvements to dental and maxillofacial implant treatments, reducing the risks of harming a patient. A series of trials has been successfully completed. The company has signed cooperation agreements with a number of leading medical research centers, including the University of Toronto. Navident became commercially available in Canada and is preparing for an international product launch in the USA and Europe.
This has been a busy year, one full of exciting changes and achievements. Navident product developments have brought major improvements to dental and maxillofacial implant treatments, reducing the risks of harming a patient. A series of trials has been successfully completed. The company has signed cooperation agreements with a number of leading medical research centers, including the University of Toronto. Navident became commercially available in Canada and is preparing for an international product launch in the USA and Europe.
While 2014 was a successful year for our company, we are excited for what 2015 holds. With the upcoming regulatory approvals, the opportunity for market expansion becomes the expectation.
Thank you to all of our customers and partners who have shared in our journey so far. We look forward to another exciting and successful year in 2015!
Sincerely,
Doron Dekel, CEO and Co-Founder
Claron Technology restructured and renamed Claronav
 On 2 January 2015 the assets of the Enterprise Imaging division of our company, Claron Technology Inc., were sold to Lexmark International for approximately $37 million in cash. Our surgical navigation division, the one responsible for Navident, remains and continues to execute our business plans uninterrupted.
On 2 January 2015 the assets of the Enterprise Imaging division of our company, Claron Technology Inc., were sold to Lexmark International for approximately $37 million in cash. Our surgical navigation division, the one responsible for Navident, remains and continues to execute our business plans uninterrupted.
The result of the transaction is a smaller company, but one that is solely focused on navigation products and is backed by substantial financial resources to guarantee its long term viability and fuel its growth. The company will continue to be led by Claron’s CEO Doron Dekel and the team working on Navident remains unchanged.
To better clarify what our company does, and to differentiate us from the “other Claron” operating under Lexmark/Perceptive, we decided to change our name to “Claronav Inc.”. The change will take effect next month (February).
UPCOMING EVENTS
IDS Cologne 2015
It is a pleasure to inform you that Claronav will be exhibiting at IDS in Cologne, 10th – 14th March.
If you plan to come, we would love to see you at our booth. We will demonstrate and let you test-drive some important new product features. We would also like to hear about your experience and discuss any suggestions you may have for us.
Meet us in Hall 2:2, Aisle A, booth 61
If you wish to meet with a specific person at Claronav, please contact Tom Tilmans and he will ensure the person will be present at the booth when you arrive.