
by ClaroNav | Sep 6, 2017 | News, Press Release
Toronto, Ontario, Canada, September 6, 2017 ClaroNav Kolahi Inc. (CKI), a subsidiary of ClaroNav, announces that NaviENT, its Surgical Navigation System, received FDA 510(K) clearance. CKI is pleased to announce that it has received FDA 510(K) clearance from the Food...
by ClaroNav | Nov 14, 2016 | News, Press Release, Uncategorised
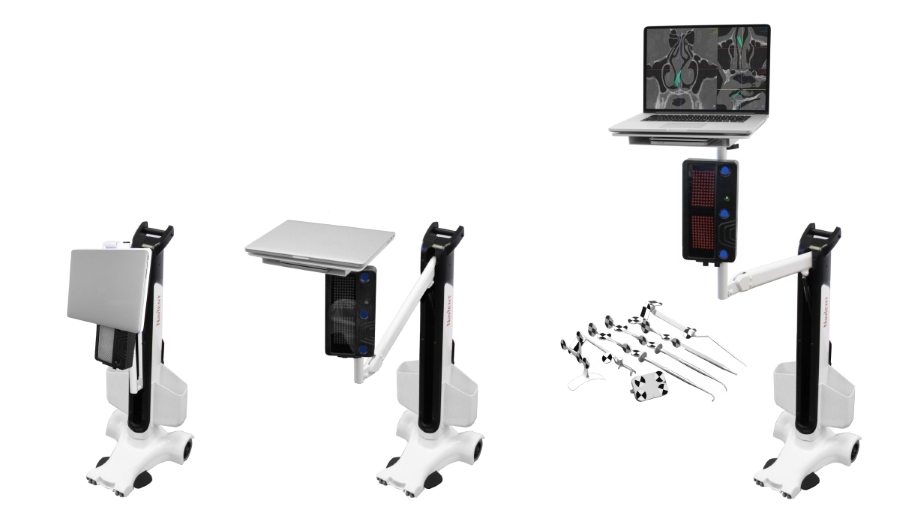
NaviENT brings Simplicity, Accuracy and Affordability to ENT Navigation Systems. Toronto, CANADA – Nov 14, 2016 ClaroNav Kolahi Inc. (CKI) announced today that it has received CE Mark approval for the commercial sale of its NaviENT cranial and ENT navigation system in...

by ClaroNav | Sep 8, 2016 | News, Press Release, Uncategorised
Toronto, Ontario, Canada, September 8, 2016 ClaroNav announces that Navident, its Dental Navigation System, received FDA 510(K) clearance. ClaroNav Inc. is pleased to announce that it has received 510(K) clearance from the Food and Drug Administration (FDA) to...
by ClaroNav | Apr 11, 2016 | News, Press Release, Uncategorised
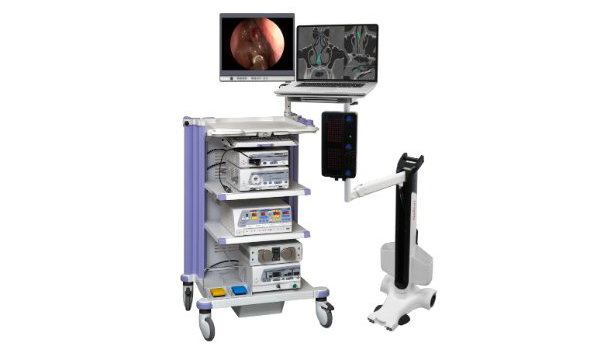
ClaroNav Inc. announced today that it has received Health Canada license for the commercial sale of its NaviENT ENT navigation system. Over the coming months the company will launch the product in ENT operating rooms, hospitals and universities throughout Canada....
by ClaroNav | Apr 27, 2015 | Press Release, Uncategorised
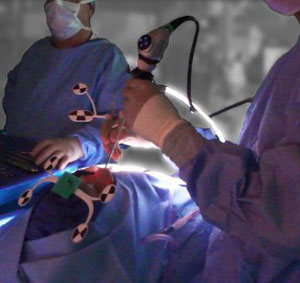
Following the sale of Claron Technology Inc.’s enterprise imaging-related assets to Lexmark in January 2015, Claron will focus its business exclusively on surgical navigation. To create a strong, independent identity for the navigation company, it has been renamed...
by ClaroNav | Apr 17, 2015 | Press Release, Uncategorised
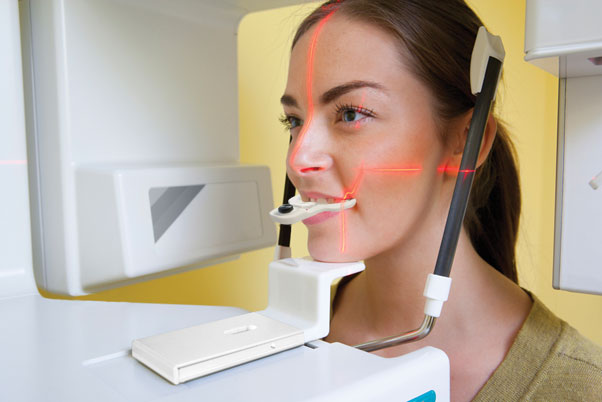
The inclusion of the CBCT scan as part of the standard of care for dental implant planning and placement has been of great benefit to the surgeon, the restorative dentist and of course to our patients. A logical next step would seem to be dynamic surgical navigation...






