
by ClaroNav | Mar 20, 2023 | Press Release
Reliable results delivered efficiently: A “Guide” to better Practice TORONTO, March 20, 2023 (GLOBE NEWSWIRE) — ClaroNav is proud to announce the launch of Navident EVO, the latest and most advanced dental navigation system available. Navident EVO is...

by ClaroNav | Nov 25, 2022 | News, Press Release
The Micron Mapper is the next generation of advanced photogrammetry. Key Benefits: Lightweight device at just over 1 lb. Allows for increased ease of use and mobility during scan capture 1 scan body for soft tissue & photogrammetry scans Simplified process...
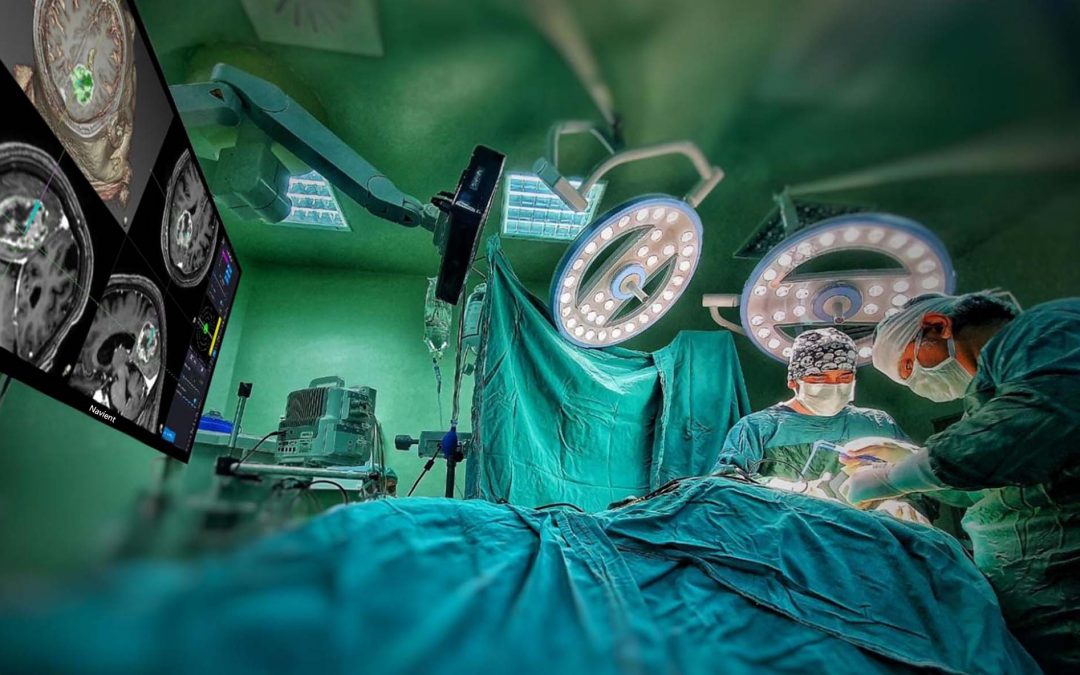
by ClaroNav | Dec 16, 2021 | News, Press Release
Navient Neuro-navigation System Receives Chinese Regulatory Clearance Dec 16, 2021 – Toronto, ON: ClaroNav Kolahi Inc, a subsidiary of ClaroNav Inc, is pleased to announce that it has received Chinese regulatory clearance from the National Medical Products...

by ClaroNav | Feb 19, 2020 | News, Press Release
TORONTO, Feb. 19, 2020 /PRNewswire/ — ClaroNav, an innovator in surgical navigation technology, announced today that it closed its first external equity financing round following five years of strong growth. The company plans to use these funds to...
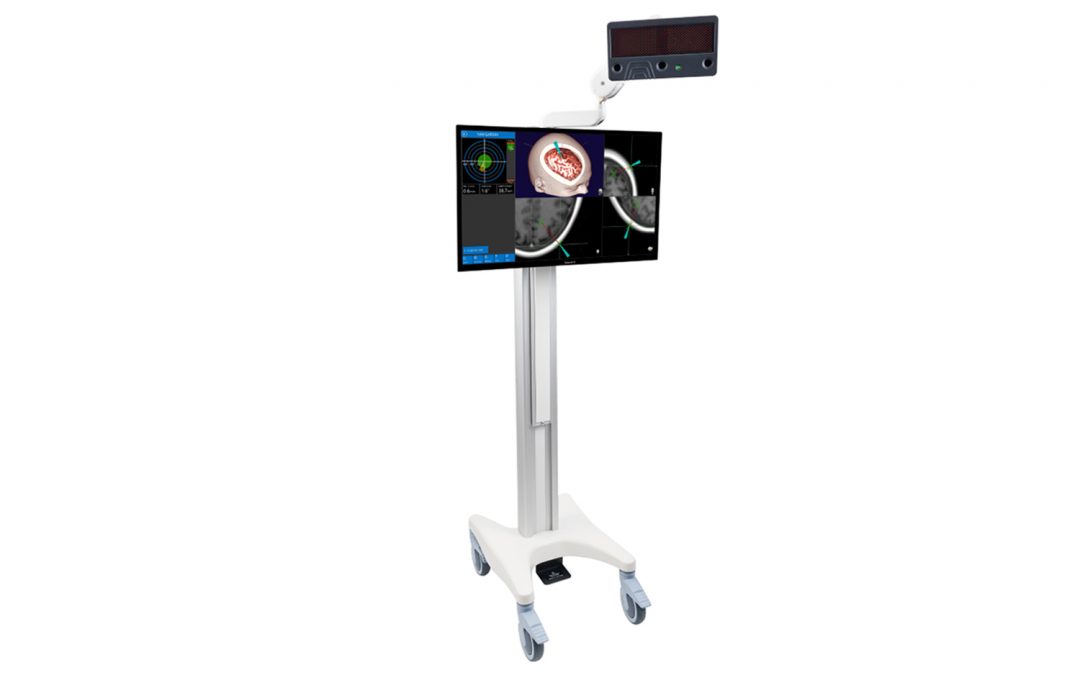
by ClaroNav | Oct 24, 2019 | Press Release
Toronto, CANADA Navient Cranial Navigation System receives CE Mark Navient brings Simplicity, Accuracy and Affordability to Cranial Navigation Systems. ClaroNav Kolahi Inc. (CKI) announced today that it has received CE Mark approval for the commercial sale of...
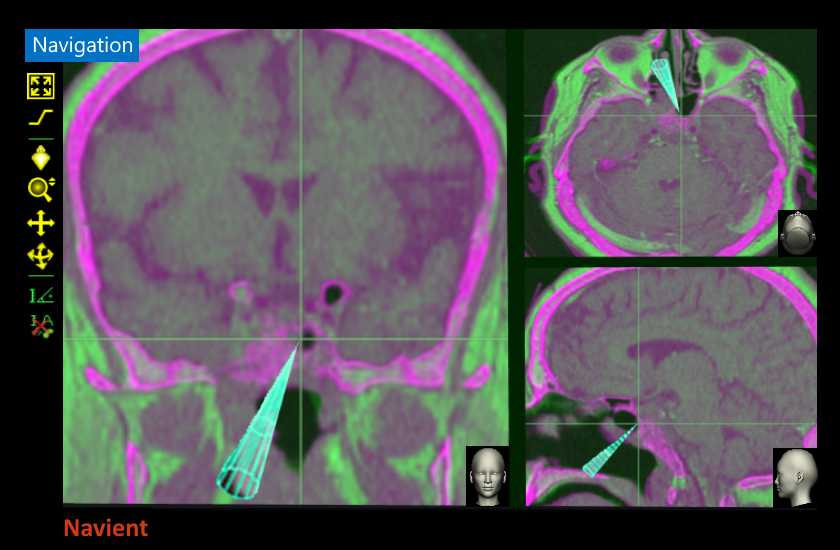
by ClaroNav | Aug 2, 2019 | News, Press Release
Toronto, Ontario, Canada August 2, 2019 ClaroNav Kolahi Inc. (CKI), a subsidiary of ClaroNav, announces that Navient, its surgical navigation system, received Health Canada approval for cranial surgery. CKI is pleased to announce that it has received...






